 |
JAPAN APPROVALS DATABASE (JAD) |
| Jouhou Koukai Publishing, a Jouhou Koukai business |
ISSN 1550-3399 |
|
|
 |
JAPAN APPROVALS DATABASE (JAD) |
| Jouhou Koukai Publishing, a Jouhou Koukai business |
ISSN 1550-3399 |
|
|
or |
The definition of Drugs Master File as stipulated by the Pharmaceutical Affairs Law:
(Drug Master File)
Article 14-11.
A person manufacturing active pharmaceutical ingredients (including the persons manufacturing in foreign countries) may register in a drug master file the matters specified by MHLW Ministerial Ordinance, including name, ingredients (in the case of an unknown ingredient - its essence), methods for manufacturing, properties, quality or storage of the active pharmaceutical ingredients.
Resources:
On April 1, 2005 the registration of MF was enforced. JKS offer two additional resources on Japanese MF:
along with other relevant documents.
 |
Reports: |
This is a JKS milestone document - describing a Manufacturing phase of the lifecycle of the regulated medicinal products in Japan. Full up-to-date description of the basics of the Drug Master Files as implemented in Japan with references and annotations. 82-page Drugs Lifecycle document. |
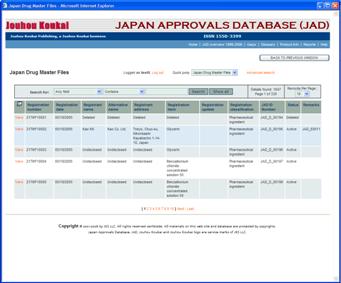 |
JAD Japan Master Files is online database containing information and data about all master files registered up to date. |