| On July 1, 2001 the Japanese Ministry of Health, Labor and Welfare introduced on voluntarily and experimental basis the acceptance of the submissions for new product approvals of Japan. After one year the experimental grace period expired and from July 1, 2003 all new submissions should be made in CTD format. JKS provide general advices on the content and the structure of the Japanese CTD (including specific modules) and on the compatibility of the format of the overseas medicinal product developer's data with the Japanese requirements. Schematic presentation of the structure of Japanese CTD is shown here
 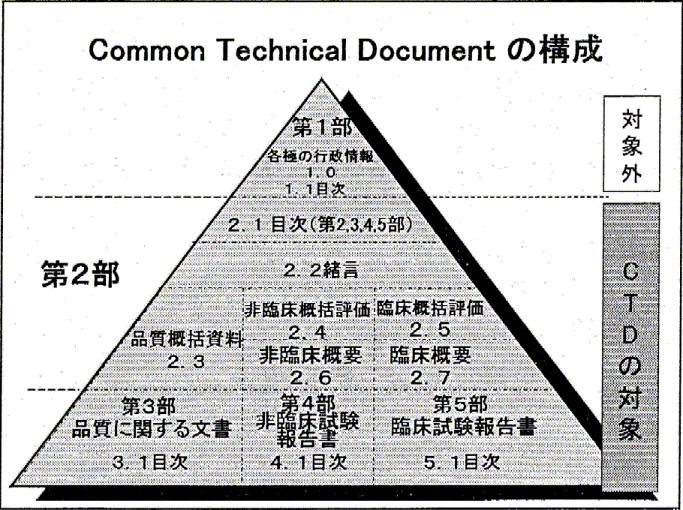 , including the Japan-specific Module 1. (Hover the mouse cursor over the thumbnail to see the enlarged image) , including the Japan-specific Module 1. (Hover the mouse cursor over the thumbnail to see the enlarged image)
Expanded description of the Japanese CTD and eCTD is available in the JKS Document Store, along with other relevant documents.
Inquire for more info...
|