December 25, 2007: MHLW: Health Policy Bureau
  announces the FY 2008 draft budget, allocates 13 billion Yen for "improvement of interferon treatment"...related reading announces the FY 2008 draft budget, allocates 13 billion Yen for "improvement of interferon treatment"...related reading
December 23, 2007: JKS News: PM Fukuda
  proposed legislation to pay uniform compensation to all eligible hepatitis C patients proposed legislation to pay uniform compensation to all eligible hepatitis C patients
December 20, 2007: JKS News: Yokohama City University
  to hold in January 2008 the International Science Forum "Scientific Tools to Promote Biological Medical Product Success" resulting from previously concluded Memorandum of Understanding to hold in January 2008 the International Science Forum "Scientific Tools to Promote Biological Medical Product Success" resulting from previously concluded Memorandum of Understanding
  with FDA Center for Biologics Evaluation and Research (CBER) with FDA Center for Biologics Evaluation and Research (CBER)
December 19, 2007: JKS News: Japan Generic Pharmaceutical Association
  to launch "Action Program for Promotion of the Safe Use of Generics" in FY 2008 to launch "Action Program for Promotion of the Safe Use of Generics" in FY 2008
December 18, 2007: JKS News: Minister of HLW
  and Minister of Finance and Minister of Finance
  agreed on draft 2008 budget calling for 1.1% cut of drug prices to reduce the healthcare burden agreed on draft 2008 budget calling for 1.1% cut of drug prices to reduce the healthcare burden
December 14, 2007: JKS News: Court   efforts to limit the financial impact on the Government, Tanabe-Mitsubishi Seiyaku KK and Benesis KK in HCV settlement rejected by plaintiffs ... research sources efforts to limit the financial impact on the Government, Tanabe-Mitsubishi Seiyaku KK and Benesis KK in HCV settlement rejected by plaintiffs ... research sources
December 13, 2007: JKS News : The Accounting Standards Board of Japan (ASBJ)
  proposes new rules for booking R&D expenses, including when drug approval is granted proposes new rules for booking R&D expenses, including when drug approval is granted
December 12, 2007: JKS News: Central Social Insurance Medical Council
  decided on the reimbursable cost for Peginterferon / Ribavirin treatment of patients with chronic hepatitis C...related reading decided on the reimbursable cost for Peginterferon / Ribavirin treatment of patients with chronic hepatitis C...related reading
December 10, 2007: JKS News: Enigmatic Eisai KK
  made MGI Pharma made MGI Pharma
  the largest ever Japanese pharma acquisition abroad...
full coverage | related readings the largest ever Japanese pharma acquisition abroad...
full coverage | related readings
December 8, 2007: National Institute of Infectious Diseases
  : comprehensive system for blood product adverse effects information gathering to be created : comprehensive system for blood product adverse effects information gathering to be created
December 7, 2007: JKS News: Kirin Holdings
  TOB for Kyowa Hakko Kogyo successfully closed...
full story | related readings TOB for Kyowa Hakko Kogyo successfully closed...
full story | related readings
December 6, 2007: JKS News: Maruho KK became the third pharma company in Japan to be awarded Hitotsubashi University Porter Prize for outstanding management ... details of company pipeline in JAD Database
December 5, 2007: JKS News: RAD-AR Council
 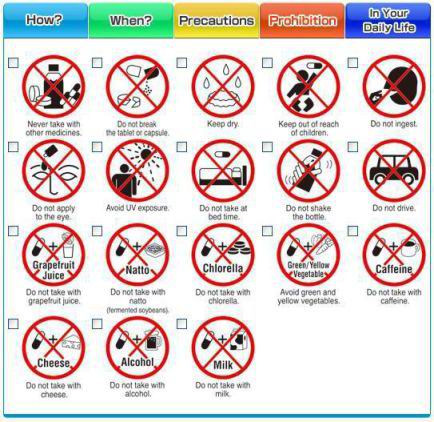 (Risk/Benefit Assessment of Drugs -- Analysis & Response Council) started testing 51 pictograms in 6 foreign languages to improve safety use of OTC drugs (Risk/Benefit Assessment of Drugs -- Analysis & Response Council) started testing 51 pictograms in 6 foreign languages to improve safety use of OTC drugs
December 5, 2007: NHK: Nearly 6% of 20-30s years old females are diagnosed with osteoporosis: lack of exercise and of Ca uptake due to dairy-deficient diet blamed
December 3, 2007: JKS News: JGPMA
  expresses great hopes for capturing larger portion of 2 trillion 800 billion yen annually pharmacy dispensing market after generics planned to be stocked in the pharmacies from April 2008 ... sources expresses great hopes for capturing larger portion of 2 trillion 800 billion yen annually pharmacy dispensing market after generics planned to be stocked in the pharmacies from April 2008 ... sources
November 30, 2007: JKS News: Daiichi Sankyo Seiyaku KK
  for the first time overtook Astellas Seiyaku to become second-largest Japanese pharma company by market capitalization ...related reading for the first time overtook Astellas Seiyaku to become second-largest Japanese pharma company by market capitalization ...related reading
November 29, 2007: JKS News: MHLW   launched massive campaign to identify HCV victims infected prior 1992 ...related reading launched massive campaign to identify HCV victims infected prior 1992 ...related reading
November 28, 2007: NKS: Drug price reduction by 1% scheduled from April 2008; medical fee disparity between hospital and private physicians to be addressed
November 21-22, 2007: Second and First Subcommittee of Drugs of PAFSC, MHLW
  pre-approved products, including 2 new anticancers ... details in JAD Database pre-approved products, including 2 new anticancers ... details in JAD Database
November 20, 2007: JKS News: Strategic Council on Intellectual Property
  proposed patent extension proposed patent extension
November 19, 2007: JKS News: MOF
  to pursue "maximum spending cuts" in 2008 budget: An advisory council of the Ministry of Finance proposed a maximum spending cuts in compiling the fiscal 2008 national budget in numerous areas such as the medical service fees and drug prices to pursue "maximum spending cuts" in 2008 budget: An advisory council of the Ministry of Finance proposed a maximum spending cuts in compiling the fiscal 2008 national budget in numerous areas such as the medical service fees and drug prices
November 18, 2007: JKS News: Japan's MHLW
  and China to initiate Asia-wide anti-cancer network ... resources and China to initiate Asia-wide anti-cancer network ... resources
November 17, 2007: JKS News: Ruling LDP
  dedicated to increase the use of generics: Mr. Yuya Miwa, a senior lawmaker from the ruling Liberal Democratic Party (LDP) - former Chairman of the LDP's Executive Council and former Minister of Health and Welfare, in the keynote presentation at the meeting of the Pharmacists Problem Studying Society announced that Government is targeting about 200 billions Yen reduction of drug-related spending, including by increasing the share of generics. An example was given by citing the large disparity in the prices for ARB class medicines - a market of the Japanese pharma market valued 400 billion Yen. dedicated to increase the use of generics: Mr. Yuya Miwa, a senior lawmaker from the ruling Liberal Democratic Party (LDP) - former Chairman of the LDP's Executive Council and former Minister of Health and Welfare, in the keynote presentation at the meeting of the Pharmacists Problem Studying Society announced that Government is targeting about 200 billions Yen reduction of drug-related spending, including by increasing the share of generics. An example was given by citing the large disparity in the prices for ARB class medicines - a market of the Japanese pharma market valued 400 billion Yen.
November 16, 2007: JKS News: J-CLIPNET
 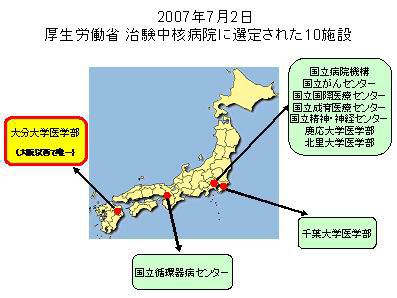 (Japan Clinical Pharmacology Network for Global Trials) established: Six leading university hospitals set up the J-CLIPNET (Japan Clinical Pharmacology Network for Global Trials) a nation-wide collaboration for early clinical trials (Proof-of-concept, POC). Its core facility is the Global Clinical Research Center (GCRC), at present having about 100 designated beds. The J-CLIPNET is a new type of academic clinical research organization aimed to became international from April 2008 by participation of similar academic facilities in S. Korea, China and The Netherlands. (Japan Clinical Pharmacology Network for Global Trials) established: Six leading university hospitals set up the J-CLIPNET (Japan Clinical Pharmacology Network for Global Trials) a nation-wide collaboration for early clinical trials (Proof-of-concept, POC). Its core facility is the Global Clinical Research Center (GCRC), at present having about 100 designated beds. The J-CLIPNET is a new type of academic clinical research organization aimed to became international from April 2008 by participation of similar academic facilities in S. Korea, China and The Netherlands.
November 15, 2007: JKS News: The Council for the Promotion of Regulatory Reform
  to propose lifting the ban on mixed treatment co-payment ... sources to propose lifting the ban on mixed treatment co-payment ... sources
November 14, 2007: JKS News: World Diabetes Day
  was observed in Japan by illuminating 15
historical and contemporary landmarks in blue was observed in Japan by illuminating 15
historical and contemporary landmarks in blue
November 12, 2007: JKS News: Sales of generics in Japan slowly increasing
  ...
full news | sources ...
full news | sources
November 10, 2007: JKS News: Cabinet Office   survey found only 27 to 39% of adults underwent cancer screening annually survey found only 27 to 39% of adults underwent cancer screening annually
November 10, 2007: NKS: Japan Patent Office   and EU, US counterparts to harmonize the screening process for biotech patents and EU, US counterparts to harmonize the screening process for biotech patents
November 9, 2007: JMA: Japan Medical Association
  no longer opposing generics to be the default Rx option from April 2008 ...
report preview no longer opposing generics to be the default Rx option from April 2008 ...
report preview
November 9, 2007: Cabinet
  : 2007 "Anti-suicide Measures White Paper" approved ...
full news : 2007 "Anti-suicide Measures White Paper" approved ...
full news
November 8, 2007: JKS News: Tokyo District Court
  found no legal basis for the government policy of restricting the mixed treatment co-payment found no legal basis for the government policy of restricting the mixed treatment co-payment
November 7, 2007: MHLW
  : Annual survey found that only 1.4% of the actually prescribed drugs in Japan are generics - much less than other estimates ...
full news |
report preview : Annual survey found that only 1.4% of the actually prescribed drugs in Japan are generics - much less than other estimates ...
full news |
report preview
November 6, 2007: JKS News: In 1978 Green Gross KK (present Tanabe-Mitsubishi Seiyaku KK) knew the HCV risk of Chrismassin   blood products ... sources blood products ... sources
November 5, 2007: JKS News: Session of the 54th general meeting of the Quality-of-Life Council held in the Prime Minister Official Residence
  ...
full news ...
full news
November 2, 2007: JKS News: PM Fukuda
  ordered legal review of Japan's laws and policies directly related to national livelihood including medicinal products ordered legal review of Japan's laws and policies directly related to national livelihood including medicinal products
October 31, 2007: MHLW
  : Prescription information revised for all SSRI and SNRI products to include warning for suicide risk in patients younger than 24 years : Prescription information revised for all SSRI and SNRI products to include warning for suicide risk in patients younger than 24 years
October 30, 2007: JKS News: Negative news on Takeda Yakuhin
  caused Tokyo Stock Exchange Pharmaceutical sector to drop by 6% caused Tokyo Stock Exchange Pharmaceutical sector to drop by 6%
October 29, 2007: JKS News: The Committee for Examination of the Use of Unapproved Medicinal Products recommended the development of 4 advanced analgesics... related info
October 29, 2007: NGMF 2007: Pfizer Chairman
  declares Asia a priority area for future development ...
related story declares Asia a priority area for future development ...
related story
October 29, 2007: NGMF 2007: Kirin Chairman Kato
  talks about "Leveraging M&A Opportunities for Quantum Leap" as a strategy for future growth...
full story | sources talks about "Leveraging M&A Opportunities for Quantum Leap" as a strategy for future growth...
full story | sources
October 28, 2007: MHLW: State to double the number of subsidized Interferon-treated HCV patients at cost of 200 billion Yen ... sources
October 25, 2007: MHLW: Minister Masuzoe
  announced at the Parliamentary hearing of the Health, Labor and Welfare Committee that the Government is
committed to solve all tainted HCV blood product cases within a year ...sources announced at the Parliamentary hearing of the Health, Labor and Welfare Committee that the Government is
committed to solve all tainted HCV blood product cases within a year ...sources
October 24, 2007: MHLW: Special Committee on Drug Prices of the Central Social Insurance Medical Council revised rules for prices of kit-consisting products ... related info
October 24, 2007: Second Subcommittee of Drugs pre-approved new products ... details in JAD Database
October 22, 2007: First Subcommittee of Drugs pre-approved new products ... details in JAD Database
October 19, 2007: MHLW: 30 new products approved in the autumn round of drug approval ... details in JAD Database
October 19, 2007: JKS News: Kirin Pharma and Kyowa Hakko in a cautionary merger ...
full news | related reading
October 18, 2007: PMDA: Two dedicated web sites opened for secure online registration of drugs and medical device recall information
October 17, 2007: PMDA: The number of Accredited Foreign Manufacturers reached 3,000 ... details in JAD Database
October 17, 2007: MHLW: Ritalin depression indication disapproved ...
report preview
October 16, 2007: JKS News: Pfizer Japan became the four-ranked domestic seller of prescription drugs ...
full news
October 15, 2007: MHLW: Japan Society for the Study of Obesity may offer less stricter criteria for metabolic syndrome ...
report preview
October 12, 2007: PMDA: East Asia Regulatory Affairs Symposium scheduled for June 2008 ... previous events
October 10, 2007: SJP: 25th New Drug Evaluation Division Information Meeting held in Tokyo ... related documents
October 10, 2007: JKS News: India's Lupin enters Japanese generics market with 10 billion Yen acquisition ...
full news
October 9, 2007: MHLW: Study Panel of the Ministry disagrees with the Japanese Urological Association on PSA-test use
October 5, 2007: MHLW: Pharmaceutical Committee gave pre-ministerial approval of new products
October 5, 2007: CSIMC: the gynecological cancer prevention and treatment system reviewed
October 2, 2007: Nippon Keizai: all listed drug development support companies reported increased earnings
October 1, 2007: MHLW: Updated version of Q&A for self-import of medications released
October 1, 2007: JKS News: Tanabe-Mitsubishi Seiyaku KK, other merged entities started new life as the fiscal year begins
October 1, 2007: PMDA: Pamphlet released and telephone line set up to facilitate the collection of postmarketing information from general public
September 27, 2007: MHLW: Study Committee for Promotion of Practice of Safety Use of Medicines held a meeting to select reference hospitals
September 27, 2007: MHLW: "Pharmaceuticals and Medical Devices Safety Information" No. 240 released
September 14, 2007: SJP: 14th Expert Meeting "Proposal for improving the analysis and evaluation of the adverse events reporting for drugs and medical devices" held
September 14, 2007: MHLW: Number of centenarians in Japan exceeds 32,000
September 13, 2007: Nippon Keizai: Largest Japanese pharmas spending 70% of profit for share buybacks
September 12, 2007: MHLW: minutes of the 2nd Vaccine Production Vision Promotional Committee meeting released
September 11, 2007: NGO: first comprehensive survey on bereaved suicide victims families completed
September 10, 2007: MHLW: new advance treatment medical institution "Hepatitis Center" (tentative name)" to be established; FY 2008 budget planned
September 8, 2007: Court ruling: State cleared in the fifth class-action lawsuit against the government and pharmaceutical manufacturers of HCV-containing blood products
September 6, 2007: Cabinet: budget framework of next fiscal year to include funds for interferon therapy of hepatitis C patients ...related reading
September 5, 2007: Nippon Keizai: most of listed Japanese bioventures are foreign owned
September 3, 2007: Yokohama City Univ: Clinical training graduate school to be established
August 31, 2007: MHLW unveiled the final version of the New Pharmaceutical Industry Vision
August 31, 2007: PMDA updated the Evaluation Guidelines for medical devices
August 29, 2007: First and Second Subcommittees of Drugs pre-approved new products, anti-influenza vaccine
August 29, 2007: MHLW: newly appointed Minister has a 3-point plan for spending cuts
August 28, 2007: MHLW: proposes to health insurance societies of large corporations to shoulder largest portion of healthcare expenses
August 27, 2007: MHLW: nation-wide dementia research project initiated, aimed to yield effective treatment within 10 years
August 21, 2007: MHLW: Japan has only 70 trained pediatric psychiatrists, most of the prefectures lacking child mental care facilities
August 20, 2007: Yomiuri Shimbun: Staff in 70% of university hospitals has been subjected to physical and verbal abuse in 2006
August 17, 2007: MHLW: Special Committee on Ethics in Clinical Trials held its first meeting
August 14, 2007: Nippon Keizai: "Golden age" of big Japanese pharma seems to be over...related reading
August 10, 2007: MHLW: Raw data of 10,000 Tamiflu patients collected, work on to analyze the causative relations with abnormal behavior started
August 8, 2007: MHLW: Next "Medical Treatment Fee Revision Basic Plan" to give priority to newly evaluated products and generics
August 7, 2007: Japanese Breast Cancer Society: in 2006 terminal cases exceeded 11,0000
August 6, 2007: MHLW: starting April 2008, generics will be a default choice in prescription forms; healthcare cost expected to drop by 1 trillion yen
August 2, 2007: Court ruling: pharmaceutical companies to compensate Hepatitis C victims
August 1, 2007: FPMAJ: Federation of Pharmaceutical Manufacturers' Associations of Japan submitted to MHLW a proposal for revising drug prices
August 1, 2007: Sanofi-aventis KK: to acquire mid-size Japanese drug maker within a year
July 31, 2007: MHLW: 17 new products approved
July 30, 2007: PMDA: Business Report for 2006 fiscal year released
July 29, 2007: National Diet: prominent AIDS activist won a seat in the Upper House elections
July 24, 2007: MHLW: in some areas of Western Japan porcine carriers of Japanese encephalitis virus exceed 80%; improved human vaccine to be available not earlier than 3 years
July 23, 2007: MHLW: draft of the New Pharmaceutical Industry Vision unveiled
July 21, 2007: Sankyo Foundation of Life Science: Fifth Sankyo Takamine Memorial Award granted for research and development into the treatment of AIDS
July 19, 2007: PMDA: dedicated OTC web site upgraded
July 13, 2007: MHLW: warning on an exploitable flaw in downloaded submission software
July 11, 2007: Microsoft Japan KK launches a panel to study the implementation of common standards for medical information system
July 9, 2007: NKS: unprecedentedly, many major insurers request their members to opt for generic drugs
July 6, 2007: Japan National Center for Suicide Prevention to conduct first nation-wide survey on victims background
July 3, 2007: NKS: listed Japanese pharma companies scared by foreign funds embarked to classical cross-shareholding
June 29, 2007: PAFSC: Pharmaceutical Affairs Department approved 2 products, recommended 13 for approval later in July
June 28, 2007: Santen Seiyaku KK, few other pharma companies responding to shareholder activists demands
June 28, 2007: PAFSC: Pharmaceutical Affairs Department to analyze the conflicts of interest in the "Key Points of Controversies for a Future Examination" plan
June 27, 2007: Central Social Insurance Medical Council: Special Committee on Drug Prices to revise the system for using foreign average price adjustment method
June 27, 2007: Central Social Insurance Medical Council: Special Committee on Drug Prices released the "Next Drug Price System Reform Main Consideration Ideas" document
June 26, 2007: Tanabe Seiyaku KK: today's shareholders' meeting cleared the
last obstacle for creation of Tanabe-Mitsubishi Seiyaku KK in October 2007...related reading
June 25, 2007: Nippon Keizai Shimbun: the cash-rich listed Japanese pharma companies re-purchasing stocks as defense against foreign take-over
June 15, 2007: Cabinet Office's White Paper on Handicapped: out of 3 million mental patients 1 million suffer from emotional, including bipolar disorders
June 15, 2007: MHLW Basic Cancer Prevention Plan: 20% reduction of cancer mortality in patients under 75; other key numerical targets included
June 13, 2007: Nippon Keizai Shimbun: second-tier pharma companies to expand R&D abroad
June 8, 2007: MHLW: aims to decrease the suicides, including depression-related by 20% within ten years
June 6, 2007: MHLW: compassionate use of unapproved drugs to be permitted in 2007
June 1, 2007: New Infectious Diseases Law enforced
May 31, 2007: MHLW: through early detection and improved treatment cancer mortality to be reduced by 20%
within ten years
May 29, 2007: 24th New Drug Evaluation Division Information Meeting: MHLW confirms the commitment to eliminate "drug lag" of 2.5 years approval time, to expand global trials
May 25, 2007: Pharmaceuticals Affairs Dept. (PAFSC): new products recommended for approval
in June
May 24, 2007: Research: 90% of suicides due to depression, mental disorders
seldom treated
May 21, 2007: MHLW: Basic Law for Cancer Prevention to be revised
May 18, 2007: Ministry of Finance: share of generics (5.2%) in Japan lowest among industrialized countries; medical devices - most expensive
May 16, 2007: MHLW to double the generics share to about 30% by fiscal year 2012
May 10, 2007: Japanese pharma cos focus on development of medicines for life style diseases
May 9, 2007: First joint clinical trial to be launched in Japan and Korea in June
April 19, 2007: MHLW to expedite drug approval process via international clinical trials
April 13, 2007: MHLW projection: number of metabolic syndrome sufferers to decrease by 10% in next 5 years
April 4, 2007: MHLW: re-examination period for drugs with new active ingredient extended to 8 years, effective April 1, 2007
March 29, 2007: MHLW panel: 128 cases of Tamiflu-related abnormal behavior documented
March 29, 2007: JPMA announced the results of "International and Joint Clinical Trials" survey
March 27, 2007: MHLW unveils "Healthcare IT Grand Design" Plan for the next 5 years
March 26, 2007: MHLW published a flu epidemic guideline reflecting recent changes in Tamiflu prescription
March 21, 2007: MHLW decides to outlaw the use of Tamiflu for 10-19 years old
March 16, 2007: MHLW made a proposal to the Council on Economic and Fiscal Policy about innovation in the medical field
March 15, 2007: PMDA Proceedings of the First International Symposium on Biologics published...more
March 7, 2007: MHLW minutes of the 11th meeting of the Committee for Examination of the Use of Unapproved Medicinal Products made public
March 6, 2007: PMDA published a draft for the Q & A on international and joint clinical trials to be adopted by mid-2007
March 2, 2007: MHLW pledges the current average 2.5 years period for review and approval to be shorten by one year within next 5 years
February 28, 2007: MHLW announced large-scale Tamiflu safety survey ...more
February 25, 2007: First and Second Subcommittees of Drugs pre-approved new products
February 16, 2007: PMDA announced new fees structure
February 13, 2007: PMDA published full report on citizens meeting on acceleration of drug and medical device development and approval
February 2, 2007: MHLW, PMDA and EC, EMEA signed confidentiality agreements ...more
January 31, 2007: Meeting between 3 ministers, industry representatives and citizens on Japanese pharma industry held
January 31, 2007: The First Subcommittee of Drugs pre-approved 7 new products
January 26, 2007: The MHLW approved 41 new products
January 25, 2007: The MHLW to extend the re-examination period for drugs with new active ingredient from 6 to 8 years
January 19, 2007: The MHLW approved the revised "Indications" in the PI of 10 cisplatin products to be used in conjunction with pemetrexed
January 10, 2007: The Committee for Examination of Issues of Clinical Trials released an interim report
January 4, 2007: MHLW approved 2 new products - mesothelioma drug Alimta became for the first time available
|





























